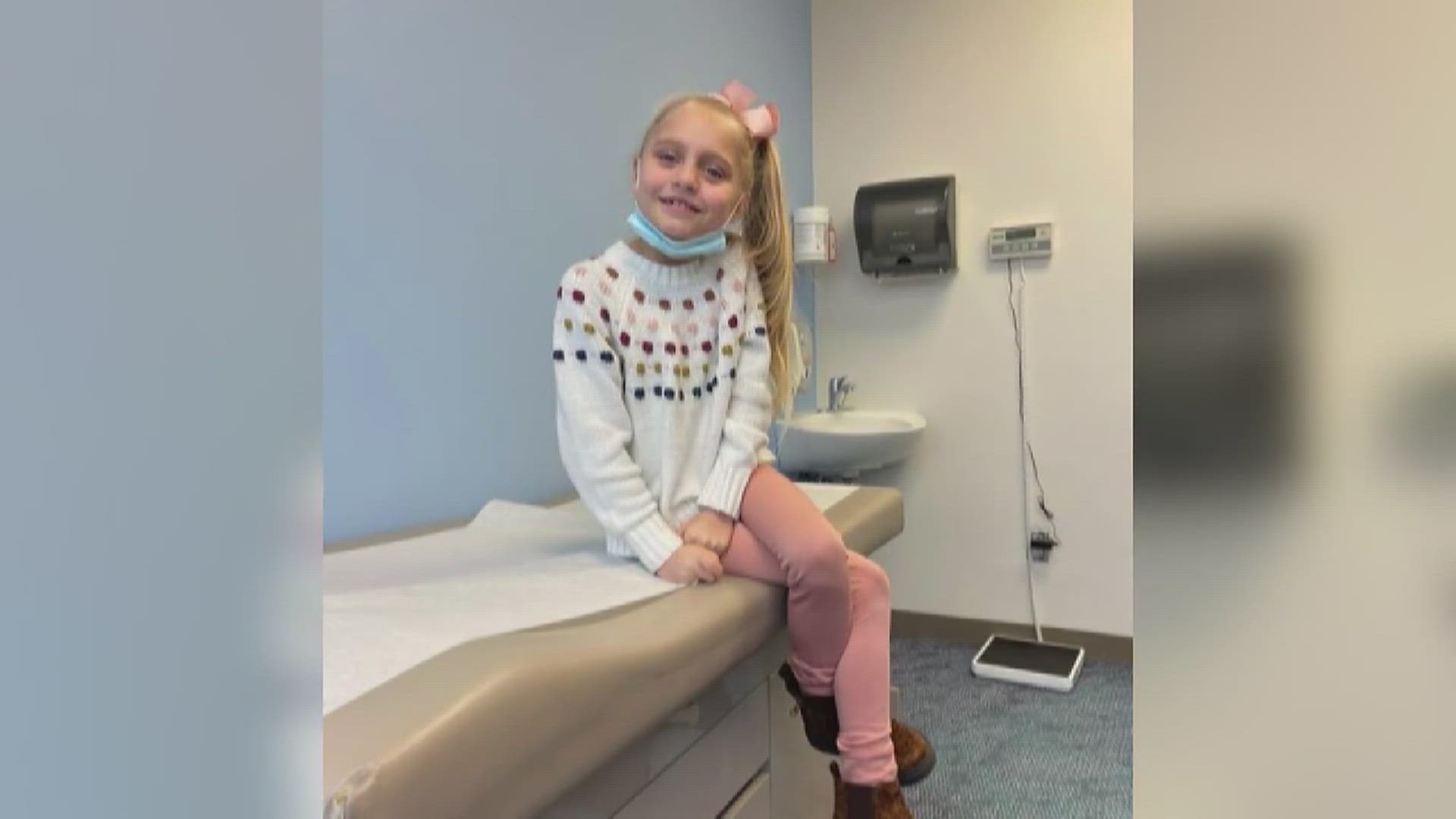
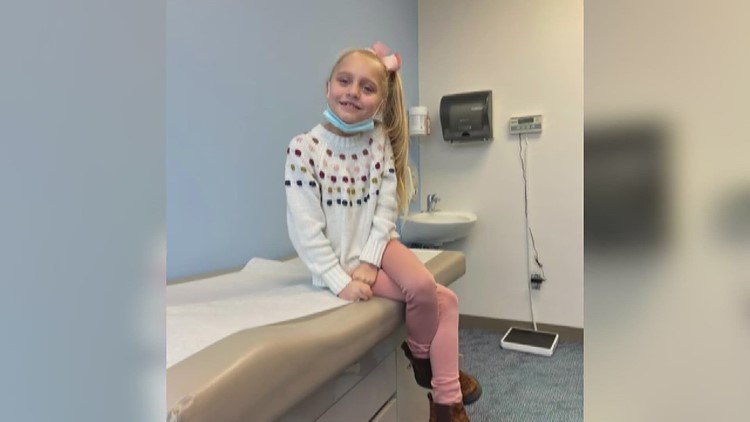
PORT NECHES, Texas — A U.S. congressman is supporting a Port Neches mother, whose 9-year-old daughter is battling a rare genetic disorder, as she demands change from the FDA.
Isla Edwards was diagnosed with CLN3 Batten disease in August 2021. The rare genetic condition has no known cure.
The illness has left Edwards with only 10 percent of her vision.
Batten-1, a proprietary liquid version of Miglustat, is the drug Edwards is taking to slow down the rare genetic disorder. It can help prevent Edward's vision from further deteriorating.
Related: Port Neches girl defying the odds, keeping hope alive as she battles rare genetic condition
The medicine is not FDA approved, is going through clinical trials and costs $18,000 per month.
Edwards could join a medical trial which would help alleviate the cost, but it comes with some risks. Fifty percent of participants will get a placebo pill.


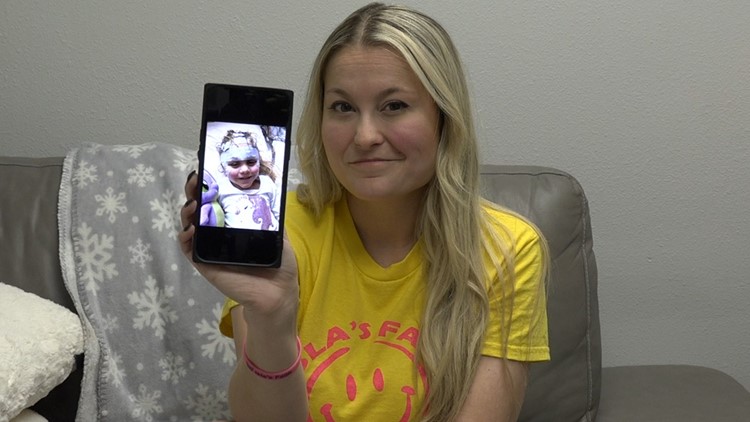
"Meaning half the kids in this final phase of the trial will have no treatment at all, which would be 35 kids that are essentially sacrificed," Jaquelyn Stockdale, Edwards’ mom, said. “We believe that these parameters are unethical and their are many other diseases that do not have placebo arms.”
As Stockdale fights for her daughter’s life, she also challenges the FDA’s use of the placebo.
“We will not put our children in a trial where they have they possibility of not getting any treatment,” Stockdale said.
Stockdale feels that no parent is willing to take the risk.
"Unfortunately, you know, this disease, the result is always the same. 100% of the time it's a terminal illness," Stockdale said. "So, why you would need a placebo arm of kids when you already know what's going to happen."
Stockdale is asking the FDA to make a change and a major voice has joined her fight. Congressman Randy Weber sent a letter to the FDA asking them to change the parameters of this particular trial.

"The FDA reconsider clinical trial designs within the population of certain fatal heterogeneous degenerative diseases so that patients do not have to choose between medication that is slowing the the progress of their diseases and participating in FDA trials," Congressman Weber said.
Stockdale could not believe the quick response coming from Congressman Weber’s office.
"Please please change the rules on this clinical trail, on this process so that placebos don't have to be involved," Congressman Weber said.
Congressman Weber said he was moved when he heard Edward's story and feels he need to give her fighting chance.
"Please reach out to a member of congress," Congressman Weber said. "Ask them to sign on to our letter. Push for Ms. Isla. She's a delightful young lady, and may I say, she doesn't deserve this."
Stockdale hopes having Congressman Weber on her side will lead to the change she and other parents are asking for.
Despite her struggles, for Isla Edwards, there are no bad days.
Anyone who would like to follow Isla Edwards' story or who wants to support her and her family can go to the Isla's Faith Facebook page or islasfaith.com.