Hospital leaders say the antibody tests help provide an extra level of surveillance, and confidence.
Right now, CHRISTUS St. Elizabeth is running a limited numbers of tests on certain patients and staff.
On Wednesday, they explained how the antibody test is different than the COVID-19 rapid test.
There are two main approaches for testing for COVID-19.
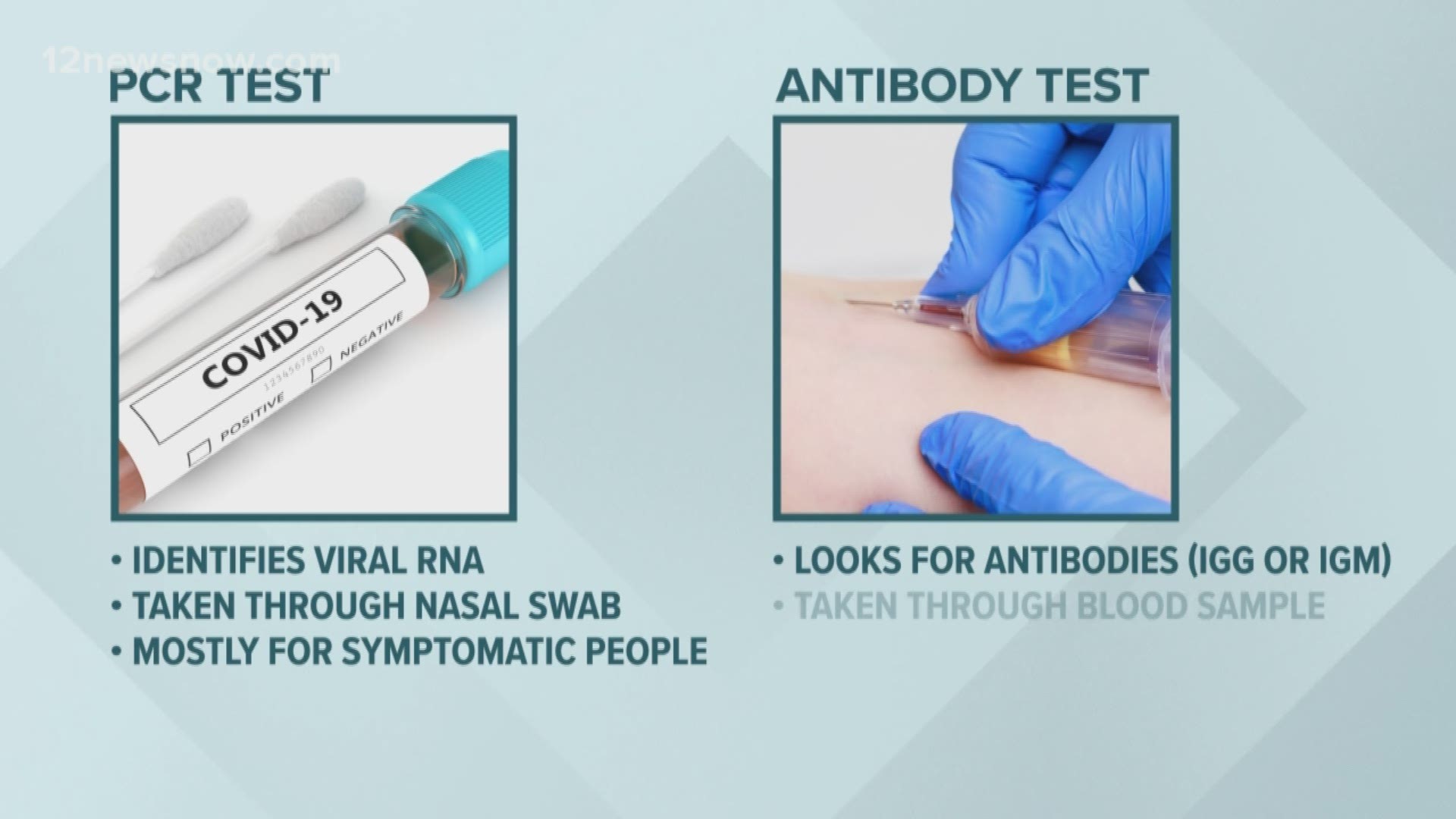
The first is the PCR tests. It's considered the 'Gold Standard' and one you're probably familiar with.
It detects the virus by identifying viral RNA. A sample is collected through a nasal swab and sent to a lab. It's primarily for people showing symptoms.
The new antibody tests don't detect the virus. It looks for antibodies that your immune system makes when it fights COVID-19.
Doctors test this with a blood sample, and believe it will eventually allow more people to be tested.
"It will provide an extra level of surveillance and confidence in our communities, CHRISTUS facility is a safe place for you to visit for your care," CHRISTUS Vice President Dave Benner said.
Right now at CHRISTUS, the antibody tests are being used on medical care providers, to help identify asymptomatic carriers. They're calling it a 'Game Changer.'
The results of the test could prevent someone from unknowingly passing the virus on.
However, the test made by Healgen Scientific has not been FDA approved.
Dr. Sam Bagchi is the chief clinical officer for CHRISTUS Health. He says they've been working with the tests for weeks.
"We've heard a lot of concerns about antibody testing and is it valid and we spent a lot of time over the last several weeks. We've had this test in house for several weeks and we've spend time validating the tests and we feel really good about the accuracy of the testing," Bagchi said.
Healgen Scientific Company has filed for an emergency use authorization.
Doctors at CHRISTUS say when an antibody test result comes back positive, a PRC test will be given to that patient to ensure the results are accurate.
If the FDA approves the test, the long term plant would be to roll out more testing sites.