AUSTIN, Texas — A Covid-19 vaccine is still weeks away from approval, but Texas Governor Greg Abbott is encouraging health care providers across the state to enroll in the DSHS immunization program.
The online portal allows hospitals, medical facilities, pharmacies and long-term care facilities to register "to be considered" to receive the Covid-19 vaccine once approved.
"While potential COVID-19 vaccines continue to undergo clinical trials, the State of Texas is taking a proactive approach to ensure the vaccine is distributed as quickly as possible once available," Governor Abbott said in a news release. "Providing Texans with access to a voluntary vaccine and efficiently administering the immunization will be essential to containing COVID-19 and protecting the health of our communities."
Supplies will be extremely limited once a vaccine is approved. Health care workers and frontline workers will be at the top of the list to be vaccinated first, according to guidance from DSHS in October.
Doses of the voluntary COVID-19 vaccine and the supplies needed to administer the immunization will be provided by the Centers for Disease Control and Prevention, according to DSHS.

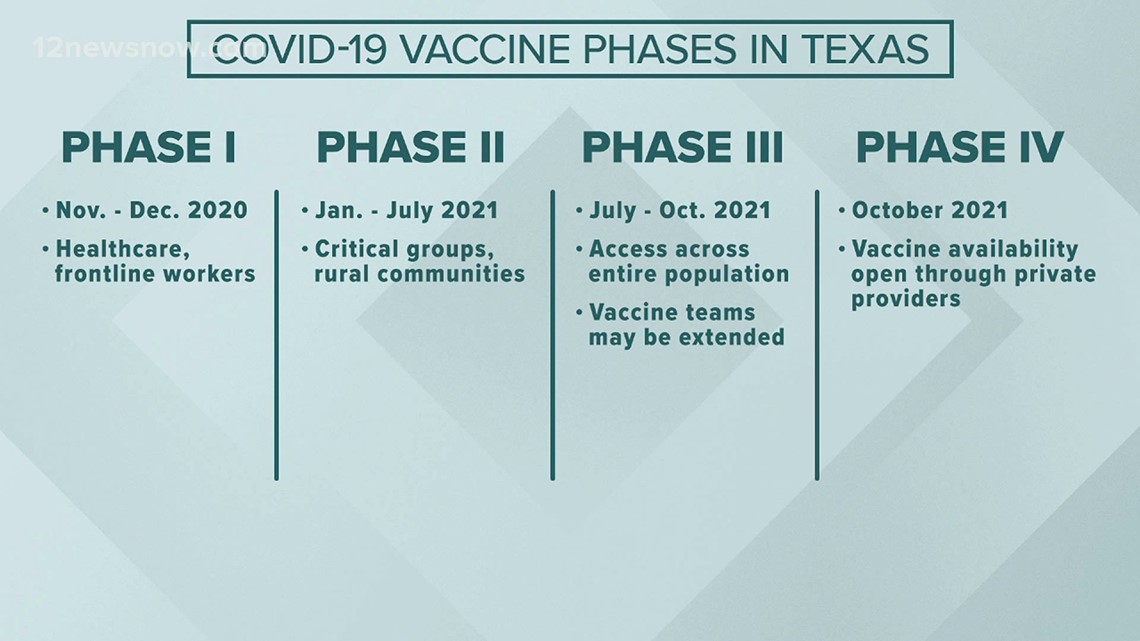
The state says that Phase II of the vaccine would include critical groups and those living in rural communities. Phase III would begin roughly six months later and would include the general population.
DSHS says that under federal guidelines, providers who choose to participate must agree to administer vaccine regardless of a recipient’s ability to pay, provide a vaccination record to each recipient, store doses of vaccine under the proper conditions, and report the number of doses received and used.
Pfizer announced on November 9 that they are in the final testing of a vaccine that has so far proved to be 90% effective. That is around the same effectiveness as the measles vaccine, and it is more effective than the standard flu shot.
It is still unclear when the Pfizer vaccine may receive emergency approval by the FDA. Several other companies are also in the final testing stage for a vaccine.

