A new 53-page document is offering new insight into the first COVID-19 vaccine up for Food and Drug Administration approval. The document analyzes Pfizer's data from its phase 3 clinical trial.
A lot is riding on this data. The FDA advisory committee will meet Thursday to discuss it. The information will be used to approve or deny emergency use authorization for Pfizer's COVID-19 vaccine.
12news investigator Lauren Hensley took the data and used it to answer some of your questions.
Question: How likely is the Pfizer vaccine to protect me from COVID-19?
Pfizer's data shows when two doses are given the vaccine is 95 percent effective in preventing symptomatic COVID-19.
Question: How effective is just one dose?
About a week after getting the first dose, the vaccine is 50 percent effective in preventing symptomatic COVID-19.
It’s important to note that this was not a trial to understand the response of a single-dose. This was an observation made while participants were waiting for that second dose.
The data shows more promising news when it comes to certain demographics. The vaccine's overall effectiveness seems to be the same across all age groups, races and even those with underlying health conditions like high blood pressure or diabetes.
But for some groups, there is not enough evidence. Pfizer says more data is needed to understand the vaccine's safety and effectiveness in children, and pregnant and breastfeeding women.
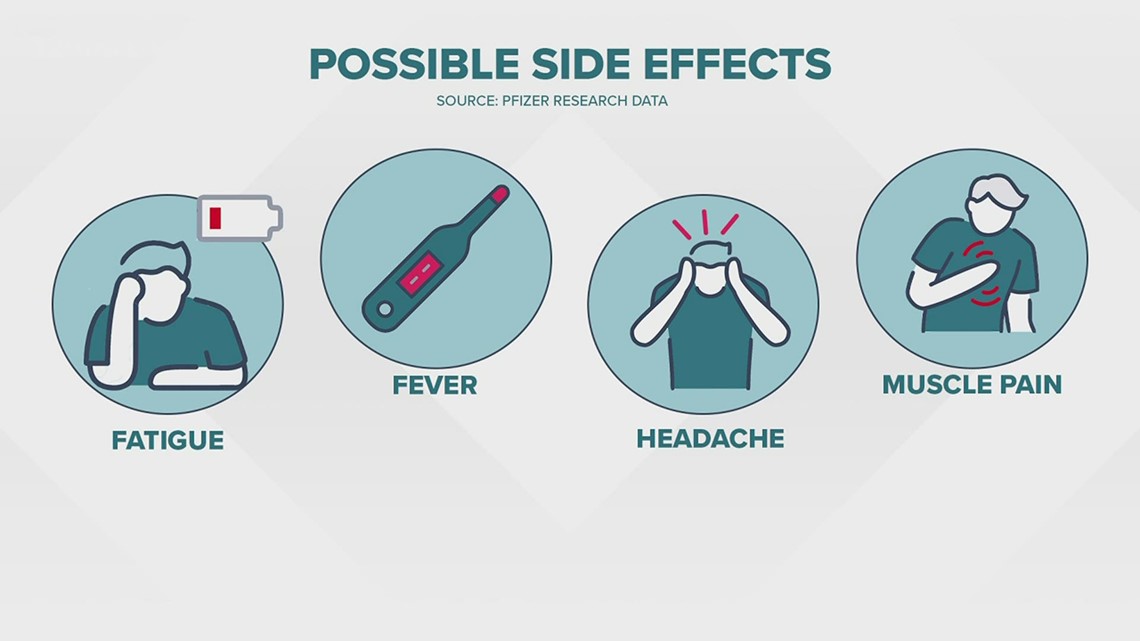
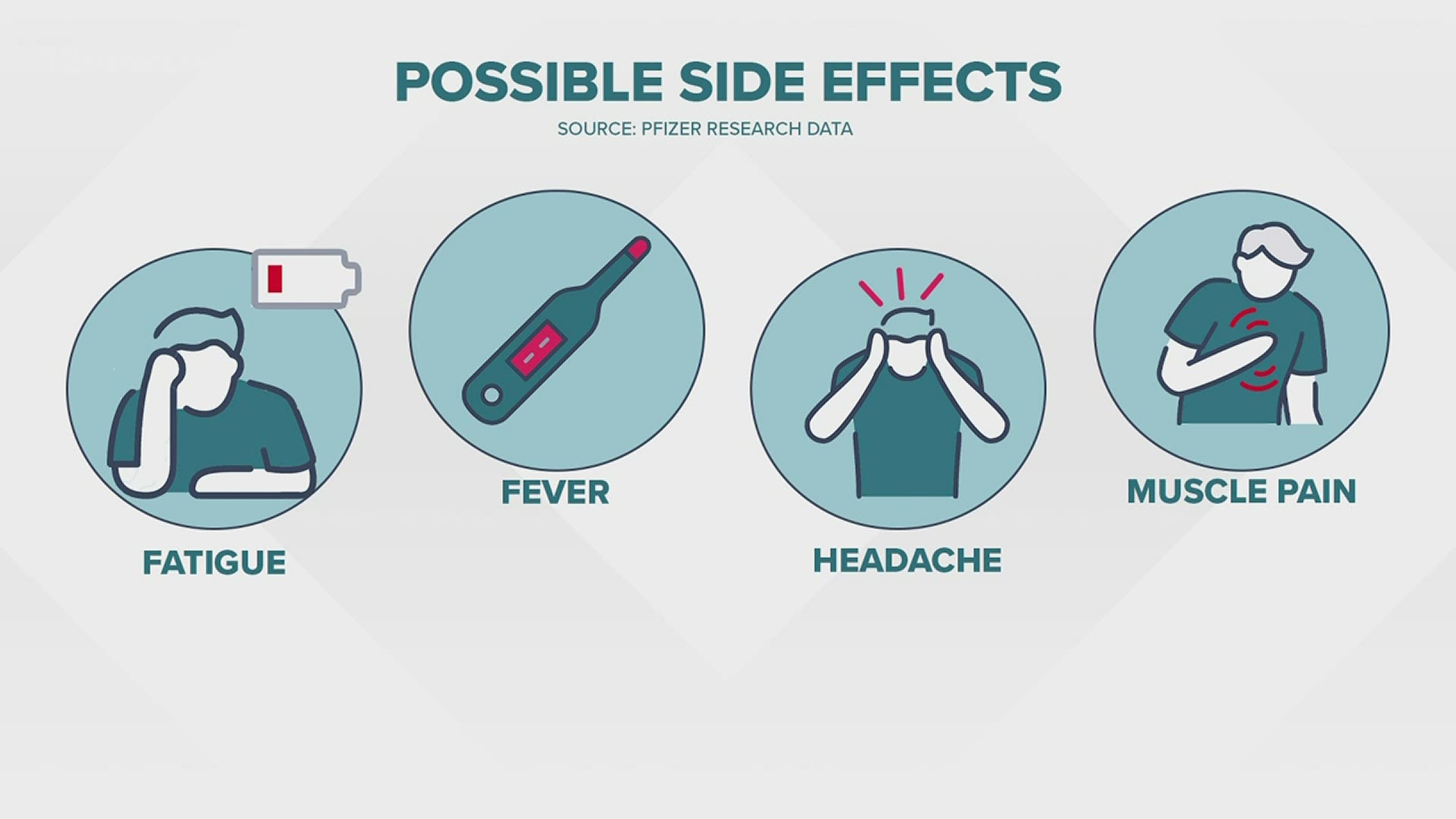
Question: Will I experience any side effects?
The most commonly reported symptoms include fatigue, fever, headaches, muscle, and joint pain.
A total of six participants died during the trials. Two people received the vaccine and four who were given a placebo. None of those deaths was linked to the vaccine.
If approved by the FDA, the first round of vaccines could be here by Monday. Those are reserved for health care workers. The general public may not see COVID-19 vaccines until April 2021.